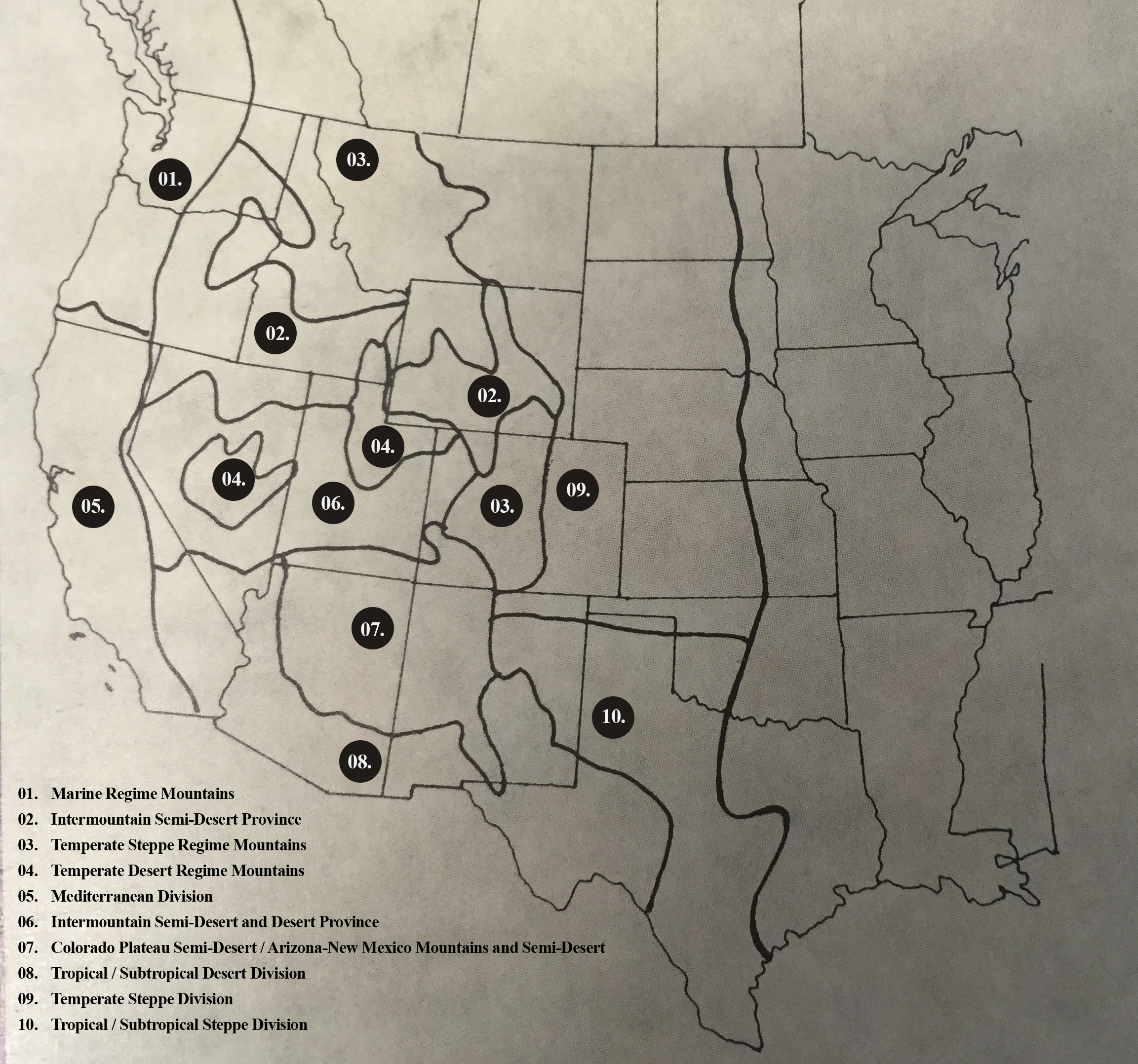
The Western Bat Species Regional Priority Matrix is a product of the Western Bat Working Group Workshop held in Reno, Nevada, February 9-13, 1998. The matrix is intended to provide states, provinces, federal land management agencies, interested organizations and individuals a better understanding of the overall status of a given bat species throughout its western North American range. Subsequently, the importance of a single region or multiple regions to the viability and conservation of each species becomes more apparent.
of contaminants on migratory bat species; information on roosting requirements, foraging ecology, and seasonal movement patterns and the need to gain a regional perspective and more complete distributional information, especially in relation to longitude, latitude, elevation, and habitat types for most species. As a means to accomplishing the latter, two groups suggested establishing a summer censusing program analogous to the Christmas bird count. As a result, a ‘National Bat Survey Week’ will be initiated by the
Western Bat Working Group with the intention of promoting the need to obtain bat data from mist netting efforts by appropriately trained researchers, managers, and biologists across the United States. The second full week of August each year will be considered ‘National Bat Survey Week.’ It will be a targeted time period for emphasis on conducting bat surveys. State bat working groups are encouraged to help promote, coordinate, and facilitate state efforts and identify locations for surveys.
The matrix should also provide a means to prioritize and focus population monitoring, research, conservation actions, and the efficient use of limited funding and resources currently devoted to bats. Research and management needs, recommended as high priority by the majority of regional analysis groups, comprise five general areas; the need for standardized sampling recognizing that population status and trend data are lacking and seriously needed for most species; monitoring the effectiveness of management actions implemented for bat conservation; assessing the effects
The Western Bat Species Regional Priority Matrix ... is intended to provide states, provinces, federal land management agencies, interested organizations and individuals a better understanding of the overall status of a given bat species throughout its western North American range.
01.
Species Priority Matrix
"High"
“High” designation represents those species considered the highest priority for funding, planning, and conservation actions. Information about status and threats to most species could result in effective conservation actions being implemented should a commitment to management exist. These species are imperiled or are at high risk of imperilment.
"Medium"
“Medium” designation indicates a level of concern that should warrant closer evaluation, more research, and conservation actions of both the species and possible threats. A lack of meaningful information is a major obstacle in adequately assessing these species’ status and should be considered a threat.
"Low"
“Low” designation indicates that most of the existing data support stable populations of the species, and that the potential for major changes in status in the near future is considered unlikely. While there may be localized concerns, the overall status of the species is believed to be secure. Conservation actions would still apply for these bats, but limited resources are best used on high and medium species.
"Periphery"
“Periphery” designation indicates a species on the edge of its range. This designation reflects neither high, medium, nor low concern.
Matrices
Tables detailing priority listings for habitat generalists and specialists
Region 1
Region 2
Regions 3, 4, 9, 10
Region 5
Region 6
Region 7, 8
Region 1
Region 2
Regions 3, 4, 9, 10
Region 5
Region 6
Region 7, 8
Region 1
Region 2
Regions 3, 4, 9, 10
Region 5
Region 6
Region 7, 8
Region 1
Region 2
Regions 3, 4, 9, 10
Region 5
Region 6
Region 7, 8
02.
Survey Matrix
Matrices
Netting
Fly low to ground and readily captured in nets, often in upland habitats.
Identification
Morphologically distinct.
Roost Location
Easy to detect colonies in man-made roosts; difficult in most natural roosts (e.g., trees and rock crevices). Frequently uses man-made roosts (e.g., mines, bridges, buildings) in parts of its range. Often found in night roosts, especially mines and bridges.
Roost Identification
Roost conspicuous, easy to identify. Guano with characteristic culled insect parts, particularly Jerusalem crickets and scorpions, often distinctive.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Subset of calls diagnostic, particularly if it gives a “directive” call.
Active Acoustic
Visually distinctive.
Netting
Effectiveness of netting depends on habitat type.
Identification
Morphologically distinct.
Roost Location
Difficult to find.
Roost Identification
Easy to detect in roost.
Passive Acoustic Detection
Difficult to detect acoustically.
Acoustic Identification
Issues currently unresolved.
Active Acoustic
Indistinguishable from Leptonycteris species, except at very close range (e.g. hummingbird feeders).
Netting
Effective at avoiding mist nets.
Identification
Morphologically similar to Idionycteris phyllotis.
Roost Location
Most effectively found by searching for colonial roosts, in mines and caves. Roosts in buildings in coastal portion of range. Some portions of range, particularly Canada and some desert areas, roosts very difficult to locate.
Roost Identification
Easy to locate and identify in roost.
Passive Acoustic Detection
Difficult to detect acoustically, low intensity calls (“whispering bat”).
Acoustic Identification
Calls, when detected, are diagnostic.
Active Acoustic
Visually distinctive in most settings.
Netting
Readily captured in mist nets, but problematic in open areas, especially where water is abundant.
Identification
Morphologically distinct.
Roost Location
Easy to locate man-made roosts; difficult to identify most natural roosts (e.g., trees and rock crevices). Natural roosts dominate throughout much of range. Night roost surveys often effective.
Roost Identification
Colonies often conspicuous, species easy to identify.
Passive Acoustic Detection
Easy.
Acoustic Identification
Subset of sequences diagnostic, acoustic overlap with Lasionycteris and Tadarida.
Active Acoustic
Visually distinctive in flight.
Netting
Can be effective where water is a limiting factor in xeric conditions, although netting is not effective in many portions of range.
Identification
Morphologically distinct.
Roost Location
Non-colonial, cliff-roosting; very difficult to locate and generally inaccessible.
Roost Identification
Unknown; no roosts have been visually inspected; only locations have been from a distance using radio-telemetry.
Passive Acoustic Detection
Easy to detect acoustically, with microphones sensitive to audible frequencies. Calls are audible to many people.
Acoustic Identification
Most sequences diagnostic, except in areas of geographic overlap with Idionycteris phyllotis.
Active Acoustic
Difficult to distinguish from I. phyllotis; otherwise, distinctive in flight.
Netting
Effectiveness of netting varies regionally. Have been netted where open flight paths are evident, or water is limiting. Forage at considerable heights; captured at drinking sites.
Identification
Morphologically distinct.
Roost Location
Most roost in cliffs and are highly inaccessible, quite frequently in building roosts. Can sometimes be found by surveying for guano and listening for loud chatter along base of cliffs.
Roost Identification
Generally requires monitoring at emergence.
Passive Acoustic Detection
Easy to detect acoustically (better with low frequency microphone). Calls in the audible range for many people.
Acoustic Identification
Calls diagnostic.
Active Acoustic
Distinctive except in areas of overlap with Eumops underwoodi.
Netting
Logistically difficult, requiring net sets over large bodies of water.
Identification
Morphologically distinct.
Roost Location
Poorly known; one study radiotracked to saguaro cactus.
Roost Identification
-
Passive Acoustic Detection
Easy to detect acoustically (better with low frequency microphone). Calls in the audible range for many people.
Acoustic Identification
Calls diagnostic except where range overlaps with Nyctinomops macrotis.
Active Acoustic
Distinctive except in areas of overlap with Eumops perotis and N. macrotis.
Netting
Captured infrequently in mist nets; demonstrates loyalty to particular water sources, but may be difficult to locate in initial surveys.
Identification
Morphologically similar to Corynorhinus townsendii.
Roost Location
Easy to detect in man-made roosts (e.g., mines); difficult in natural roosts (e.g., trees, rock crevices).
Roost Identification
Easy, roost in clusters on open surface (e.g., domes of mines). May be confused with C. townsendii.
Passive Acoustic Detection
Easy to detect acoustically (with low frequency microphone).
Acoustic Identification
Most sequences diagnostic, except can be difficult to distinguish from Euderma maculatum. Geographic overlap with E. maculatum throughout much of its range. Highly distinctive social call.
Active Acoustic
Can be difficult to distinguish from E. maculatum.
Netting
Vulnerability to net capture varies with habitat, but generally quite susceptible to capture. Captured over water sources, large and small.
Identification
Morphologically distinct.
Roost Location
Very difficult to locate in natural roosts (e.g. trees and snags).
Roost Identification
Unlikely to locate via roost search, but can be distinguished visually in flight upon exit.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Some calls distinctive, but overlap with Tadarida and Eptesicus. In areas without Tadarida, many sequences are diagnostic.
Active Acoustic
With experience can be distinguished visually in flight.
Netting
Sometimes captured in mist nets, but foraging areas often not suitable for netting (e.g., over large water sources).
Identification
Morphologically distinct except where overlaps with Lasiurus borealis.
Roost Location
Non-colonial. Very difficult to locate tree roosts.
Roost Identification
Difficult to locate bats in foliage, easy to identify except where overlaps with L. borealis.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Most sequences diagnostic, in areas without L. borealis. In areas with L. borealis, extensive acoustic overlap, but probably distinguishable statistically. Some acoustic overlap with Parastrellus hesperus.
Active Acoustic
Distinctive in flight except in areas with L. borealis.
Netting
Fly high; often under-represented in net captures. Often forages in areas that cannot be feasibly netted.
Identification
Morphologically distinct.
Roost Location
Non-colonial. Very difficult to locate tree roosts.
Roost Identification
Difficult to locate bats in foliage, but easy to distinguish from other species.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Many calls diagnostic throughout much of its range; subset of calls overlap with Tadarida and Nyctinomops femorosacccus.
Active Acoustic
Distinctive in flight.
Netting
Readily captured in some habitats; apparently difficult in others. Not enough known about appropriate habitats.
Identification
Morphologically distinct.
Roost Location
Difficult to locate tree roosts. Can sometimes be located by monitoring palm trees at emergence time.
Roost Identification
Difficult to observe in roost, but easy to identify during emergence from roost.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Most sequences diagnostic, but some acoustic overlap with Lasiurus borealis and Eptesicus fuscus.
Active Acoustic
Reasonably distinctive in flight.
Netting
Effectiveness of netting depends on habitat type.
Identification
Morphologically distinct.
Roost Location
Roosts in mines and caves; highly colonial.
Roost Identification
Easy to detect and identify in roost except in areas of overlap with Leptonycteris nivalis.
Passive Acoustic Detection
Difficult to detect acoustically.
Acoustic Identification
Issues currently unresolved.
Active Acoustic
Indistinguishable in flight from L. nivalis and Choeronycteris, except possibly at very close range (e.g. hummingbird feeders).
Netting
Effectiveness of netting depends on habitat type.
Identification
Morphologically distinct.
Roost Location
Roosts in mines and caves; colonial.
Roost Identification
Easy to locate and identify, except in areas of overlap with Leptonycteris curasoae.
Passive Acoustic Detection
Unknown, but presumably difficult to detect acoustically.
Acoustic Identification
Issues currently unresolved.
Active Acoustic
Indistinguishable in flight from L. curasoae and Choeronycteris, except possibly at very close range (e.g. hummingbird feeders).
Netting
Avoids mist nets.
Identification
Morphologically distinct.
Roost Location
Most effectively found by searching for colonial roosts, primarily in mines and caves.
Roost Identification
Easy to locate and identify in roost.
Passive Acoustic Detection
Difficult to detect acoustically.
Acoustic Identification
Subset of calls diagnostic.
Active Acoustic
Can identify visually at close range.
Netting
Readily captured in nets, but very delicate and often die. Suggest using harp traps.
Identification
Morphologically distinct.
Roost Location
Roosts in caves.
Roost Identification
Presumably easy to locate and identify when present.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Calls highly diagnostic.
Active Acoustic
So distinctive acoustically that visual observation does not contribute to identification.
Netting
Readily captured in mist nets.
Identification
Morphologically distinct except where range overlaps with Myotis evotis.
Roost Location
Easy to detect in man-made roosts; difficult in most natural roosts. Likely that natural roosts dominate.
Roost Identification
Roost in small groups. Requires handling for positive identification.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Probably many sequences diagnostic except in area of geographic overlap with M. evotis.
Active Acoustic
Visual cues will not help distinguish from M. evotis.
Netting
Readily captured in mist nets.
Identification
Morphologically similar to Myotis ciliolabrum. Can be distinguished from M. ciliolabrum by combination of capture and recording of hand-release echolocation call.
Roost Location
Can be found in man-made roosts, but generally non-colonial and crevice-roosting; most roosts not man-made and difficult to find. Sometimes found in night roosts.
Roost Identification
Requires handling for positive identification.
Passive Acoustic Detection
Easy.
Acoustic Identification
Difficult to distinguish from Myotis yumanensis.
Active Acoustic
Flight behavior distinguishes it from M. yumanensis in most settings.
Netting
Readily captured in nets in some portions of its range, but vulnerability to netting may vary regionally.
Identification
Morphologically similar to Myotis californicus. Can be reliably identified using combination of morphological and acoustic data.
Roost Location
Predominantly non-colonial. Frequently inhabits mines, but natural roosts likely dominate, and difficult to find. Sometimes found in night roosts.
Roost Identification
Roost in small groups. Requires handling for positive identification.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Not currently distinguishable from other 40 kH Myotis
Active Acoustic
Can sometimes be distinguished when observed in flight, but requires experience.
Netting
Readily captured in mist nets at both aquatic and terrestrial sites.
Identification
Morphologically distinct except in areas of overlap with Myotis auriculus, M. keenii, or M. septentrionalis. Also similarity to M. thysanodes in some regions.
Roost Location
Can be detected in man-made roosts, but often cryptic; difficult in most natural roosts (e.g., trees and rock crevices). Natural roosts dominate. Sometimes in night roosts, particularly mines and bridges, although extent to which these features are used varies regionally.
Roost Identification
Small colonies. Generally crevice roosting. Often requires handling for positive identification.
Passive Acoustic Detection
Intermediate intensity calls.
Acoustic Identification
Subset of sequences diagnostic except in area of geographic overlap with M. auriculus, M. septentrionalis or possibly M. keenii. Also possible confusion under some habitat conditions with 40 kHz Myotis.
Active Acoustic
May be helpful in distinguishing it from short-eared Myotis.
Netting
Difficult to find. Most netting records from known cave roosts.
Identification
Issues currently unresolved, but probably difficult to distinguish from M. evotis. Due to uncertainties regarding identification, morphometric data, hand-release calls, and wing-biopsy should be collected from all individuals.
Roost Location
Can be detected in caves and buildings, but difficult in tree roosts. Tree roosts probably dominate.
Roost Identification
Small colonies and difficult to distinguish from M. evotis. Often requires handling for positive identification.
Passive Acoustic Detection
Presumably has intensity similar to M. evotis.
Acoustic Identification
Issues currently unresolved, but likely difficult to distinguish from M. evotis.
Active Acoustic
Unknown.
Netting
Readily netted in some areas; net-avoidance in others.
Identification
Morphologically similar to Myotis yumanensis and M. occultus. Can be reliably identified using combination of morphological and acoustic data.
Roost Location
Frequently in man-made roosts (e.g., mines, bridges, buildings) in parts of its range. Difficult to find in most natural roosts (e.g., trees and rock crevices). Sometimes found in night roosts.
Roost Identification
Highly colonial and easy to detect in man-made roosts. Often requires handling for positive identification.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Some calls/sequences diagnostic, though probably not distinguishable from M. occultus in areas of geographic overlap. Difficult to distinguish from other 40 kH Myotis.
Active Acoustic
Flight behavior sometimes distinctive, particularly over water.
Netting
Fairly easy to capture in nets.
Identification
May be difficult to distinguish from Myotis lucifugus in areas of overlap.
Roost Location
Roost in man-made roosts, but natural roosts dominate. Can often be found in night roosts.
Roost Identification
Easy to detect in man-made roosts; difficult in most natural roosts. Often requires handling for positive identification.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Issues currently unresolved but probably difficult to distinguish acoustically from other 40 kH Myotis.
Active Acoustic
Difficult to distinguish visually.
Netting
More successful in interior forest than over water in eastern deciduous forest; harp traps set in gaps between trees effective in South Dakota and Wyoming. Occasionally captured over water.
Identification
Easy except where range overlaps with M. evotis.
Roost Location
Surveys for night roosts and hibernacula can be effective; day roosts under bark.
Roost Identification
Very cryptic in day roosts. Requires handling for positive identification.
Passive Acoustic Detection
Intermediate intensity calls.
Acoustic Identification
Many sequences diagnostic, but overlap with other 40kH Myotis, particularly M. lucifugus. Also potential for confusion with M. evotis.
Active Acoustic
May be helpful in distinguishing it from small-eared Myotis. Often flies in cluttered settings where identification can be difficult.
Netting
Readily captured in mist nets (often on secondary streams in northwestern portion of ran
Identification
Generally easy, but morphologically similar to M. evotis in some regions.
Roost Location
Can be detected in man-made roosts, but difficult in most natural roosts (e.g., trees and rock crevices). Natural roosts dominate. Sometimes found in night roosts.
Roost Identification
Small colonies and often in crevices. Requires handling for positive identification.
Passive Acoustic Detection
Intermediate intensity calls.
Acoustic Identification
Many sequences/calls diagnostic. Possible confusion with Antrozous pallidus.
Active Acoustic
Flight behavior, in combination with call morphology, sometimes helpful.
Netting
Limited usefulness in some habitats.
Identification
Morphologically distinct, but potentially confused with Myotis occultus or M. lucifugus.
Roost Location
Primarily in caves and rock crevices, but occasionally in buildlings.
Roost Identification
Roost colonially; can be confused with other colonially roosting Myotis and Eptesicus fuscus.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Overlap with other 40 kHz Myotis. Acoustic identification best in areas without other 40 kHz Myotis.
Active Acoustic
Visually similar to other 40 kHz Myotis.
Netting
Effectiveness of netting varies regionally, and setting makes a difference.
Identification
Morphologically distinct.
Roost Location
Can be found in man-made roosts; difficult in most natural roosts. Natural roosts dominate. Often found in night roosts.
Roost Identification
Requires handling for positive identification.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Issues currently unresolved with other 40 kH Myotis.
Active Acoustic
Flight behavior can be distinctive, i.e., long tail membrane.
Netting
Water-skimming foraging style makes this species highly vulnerable to capture in mist-nets set over still water.
Identification
Morphologically similar to M. lucifugus and M. occultus. Can be distinguished from these two species by a combination of capture and recording of hand-release echolocation call.
Roost Location
Commonly in man-made roosts. Form large aggregations in night roosts, particularly bridges. Difficult to locate most natural roosts.
Roost Identification
Highly colonial and easy to detect in man-made roosts. Requires handling for positive identification.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Difficult to distinguish from M. californicus, though some calls diagnostic (50kH Myotis).
Active Acoustic
Flight behavior, particularly water skimming, distinctive.
Netting
Effective in low-elevation canyon sites, and near known roosts.
Identification
Morphologically distinct, but potentially confused with Tadarida brasiliensis.
Roost Location
Roosts often inaccessible. Roosts primarily in cliffs. Sometimes possible to find roosts by surveying for guano and listening for chatter at base of cliffs.
Roost Identification
Generally requires monitoring at emergence.
Passive Acoustic Detection
Easy to detect acoustically, calls in the audible range for many people.
Acoustic Identification
Subset of calls/sequences diagnostic, some overlap with both Tadarida and Lasiurus cinereus.
Active Acoustic
Useful for distinguishing from L. cinereus.
Netting
Records extremely limited suggesting serious challenges.
Identification
Morphologically distinct.
Roost Location
Generally cliffs and rock crevices; often inaccessible. Also known to use building and tree roosts. Guano deposits and chatter can potentially be used to locate roosts, but generally not effective.
Roost Identification
Generally requires monitoring at emergence.
Passive Acoustic Detection
Easy to detect acoustically (best with low frequency microphone), calls in audible range for some people.
Acoustic Identification
Most calls diagnostic, but overlap with Eumops underwoodi and possibly E. perotis. Species poorly known.
Active Acoustic
Indistinguishable from Eumops in flight.
Netting
Captured in nets fairly readily, although often fly high.
Identification
Morphologically distinct.
Roost Location
Predominantly cliff-roosting. Some roosting in man-made structures, particularly mines.
Roost Identification
Usually non-colonial or small colonies. Can be identified visually at very close range.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Most calls diagnostic, although some overlap with Lasiurus blossevillii.
Active Acoustic
Visually distinctive.
Netting
While sometimes captured in mist nets, this species flies high and is generally more abundant than net captures would suggest.
Identification
Generally distinctive, but potentially confused with Nyctinomops femorosaccus.
Roost Location
Highly colonial and easy to detect in man-made roosts; difficult in most natural roosts. Natural roosts (e.g., cliff roosts) dominate in large portion of range. Commonly in man-made roosts in portion of its range.
Roost Identification
Easy to locate and identify in most roosts. Guano and odor distinctive.
Passive Acoustic Detection
Easy to detect acoustically.
Acoustic Identification
Some calls overlap with other species (Lasionysteris, Eptesicus, L. cinereus, N. femorosaccus), but fair proportion are diagnostic. In most settings this would be the easiest way to detect the species.
Active Acoustic
Visually distinctive except where overlaps with N. femorosaccus.
